1. Which of the following dry cells is a secondary cell?
A Zinc-carbon cell
B Nickel-cadmium cell
C Silver oxide cell
D Alkaline manganese cell
2. Which of the following substances can make a chemical cell?
A Zinc, plastic rod and magnesium chloride solution
B Zinc, iron and tetrachloromethane
C Magnesium, zinc and sodium nitrate solution
D Copper, graphite and water
3.
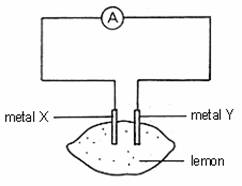
Which of the following pairs of metals would give the greatest reading on the ammeter?
Metal X Metal
Y
A Magnesium Silver
B Copper Zinc
C Silver Copper
D Iron Lead
4.
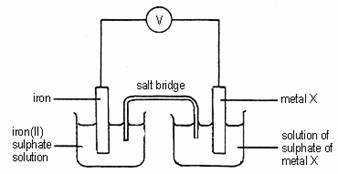
When metal X is connected to iron in the above cell, the mass of metal X increases. Metal X may be
A silver.
B zinc.
C iron.
D magnesium .
5. Which of the following statements concerning a chemical cell is/are correct?
(1) Oxidation occurs at the anode.
(2) Reduction occurs at negative electrode.
(3) Electrons flow through the electrolyte.
A (1) only
B (3) only
C (1) and (2) only
D (2) and (3) only
6.
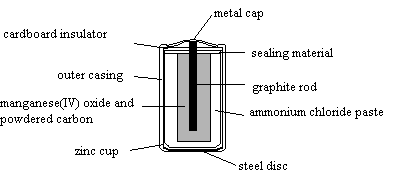
The positive electrode of the cell is
A. powdered carbon and manganese(IV) oxide.
B. zinc.
C. metal cap.
D. graphite rod.
7.
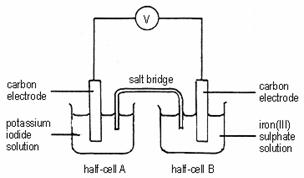
Which of the following statements concerning the above set-up is INCORRECT?
A Electrons flow from half-cell B to half-cell A through the external circuit.
B The solution in half-cell A becomes brown in colour.
C The colour of the solution in half-cell B changes from yellow to green.
D Oxidation occurs in half-cell A.
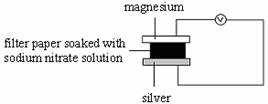
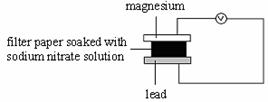
8. Which of the following set-ups will give the greatest deflection on the voltmeter?
A B
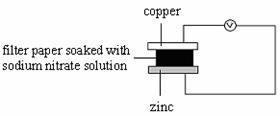
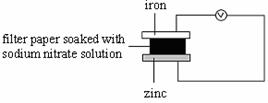
C D
9.
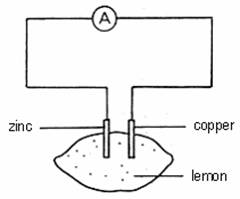
Which of the following statements concerning the above chemical cell is INCORRECT?
A The lemon juice acts as an electrolyte.
B The ammeter shows a deflection.
C The lemon juice contains mobile electrons.
D Zinc electrode loses electrons to form zinc ions.
Directions: Questions 10-11 refer to the mercury electrolytic cell shown below.
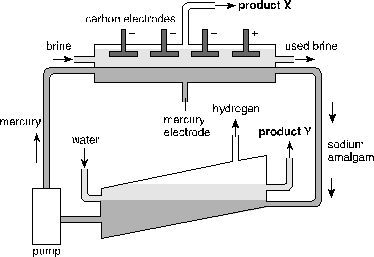
10. Which of the following statements is correct?
A. Product X is hydrogen.
B. The mercury electrode is the anode.
C. Sodium is produced at the mercury electrode.
D. The used brine contains a lot of oxygen.
11. Product Y is
A. chlorine.
B. chlorine amalgam.
C. sodium amalgam.
D. sodium hydroxide.
12. Which of the following substances CANNOT be found in a silver oxide cell?
A. Ammonium chloride
B. Potassium hydroxide
C. Silver oxide
D. Zinc
13. Which of the following chemicals is used as electrolyte in an alkaline manganese cell?
A. Ammonium chloride
B. Ammonium hydroxide
C. Potassium hydroxide
D. Sodium hydroxide
Directions:Questions 14 to 16 refer to the following set-ups. A piece of filter paper moistened with sodium chloride solution is put between the two metals in each case.
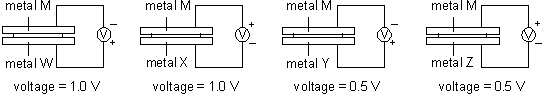
14. Which of the following represents the order of tendency to form ions of the metals?
A. W > Y > M > X > Z
B. X > M > W > Z > Y
C. X > Z > M > Y > W
D. Y > X > Z > M > W
15. In which of the following cases would you expect a reaction to occur?
A.  B.
B. 
C.  D.
D. 
16. Which of the two metals used as electrodes would give the highest cell voltage?
A. M and W
B. X and W
C. X and Y
D. M and Z
17.
|
Cell |
W |
X |
Y |
Z |
|
Ability to give steady voltage |
Excellent |
Good |
Good |
Poor |
|
Service life |
Long |
Long |
Long |
Short |
|
Shape |
Button |
Cubic |
Cylindrical |
Cylindrical |
Which of the above cells may be a zinc-carbon cell?
A W
B X
C Y
D Z
18. Electric currents are carried through sodium chloride solution by the movement of
A atoms.
B molecules.
C ions.
D electrons.
19. Consider the following chemical cells.
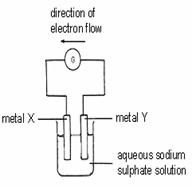
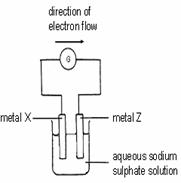
Which of the following represents the correct order of reactivity of metals X, Y and Z?
A X>Y>Z
B Y>X>Z
C Y>Z>X
D Z>X>Y
20. Which of the following dry cells is/are primary cell(s)?
(1) Silver oxide cell
(2) Nickel-cadmium cell
(3) Alkaline manganese cell
A (2) only
B (3) only
C (1) and (2) only
D (1) and (3) only
21.
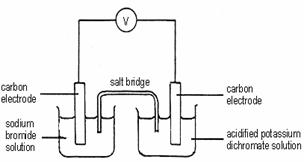
Which of the following combinations is correct?
Oxidizing
agent Reducing
agent
A Dichromate ion Sodium ion
B Bromide ion Dichromate ion
C Dichromate ion Bromide ion
D Bromide ion Potassium ion
22. Which of the following statements concerning the following set-up are correct?
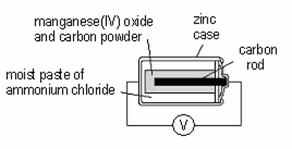
(1) Electrons flow from the zinc case to the carbon rod in the external circuit.
(2) The zinc case becomes thinner.
(3) Ammonium chloride is oxidized.
A (1) and (2) only
B (1) and (3) only
C (2) and (3) only
D (1), (2) and (3)
23. In the set-up shown below metal Y forms ions more readily than metal X.

Which of the following statements concerning this set-up is correct?
A. Electrolysis occurs inside the lemon.
B. Chemical energy is changed into electrical energy.
C. The mass of metal X decreases.
D. Electrons flow from metal X to metal Y in the external circuit.
24. Which of the following statements concerning a salt bridge is/are correct?
(1) It is prepared by moistening filter paper with distilled water.
(2) It provides ions to balance the charges in the solution of two half cells.
(3) It allows the movement of electrons between two half cells.
A. (1) only B. (2) only
C. (1) and (3) only D. (2) and (3) only
25. Consider the following chemical cell:
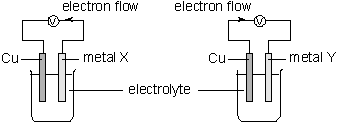
From the above information, which of the following statements is correct for a chemical cell set
up between metal X and metal Y?
A. Metal Y is oxidized.
B. Metal X is the positive electrode.
C. No current flow is detected.
D. Electrons flow from metal X to metal Y through the external circuit.
26. In which of the following cells will the bulb shine?

A. (1) only
B. (2) only
C. (1) and (3) only
D. (2) and (3) only
27. Which of the following statements concerning an alkaline manganese dry cell are correct?
(1) A larger dry cell gives a higher voltage.
(2) The electrolyte is potassium hydroxide.
(3) It is a device to change chemical energy into electrical energy.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
|
|
1st statement |
2nd statement |
|
28.. |
A zinc-carbon cell leaks after prolonged usage. |
The thickness of the zinc case decreases during discharge. |
|
29. |
The zinc case of a
zinc-carbon cell becomes thicker when it has been used for a long time. |
The zinc ions gain electrons to form zinc metal. |
|
30. |
Dry cells should not be put into a fire. |
Dry cells may explode when subjected to intense heat. |